Packaging for Sterilization
Choosing sterilization wrappers is a critical step in the sterilization process and requires knowledge of reusable and single-use wraps, their intended use, and how to seal and label them. These next few slides will introduce you to different types of single-use wrapping products and container systems used for the packaging of equipment and instruments for sterilization.
Packaging is critical to sterilization and inventory processes, as the use of inappropriate packaging materials and techniques can lead to sterilization failure. Improper handling and storage of sterile products can damage wrappers and impair the integrity of the contents.
Event-Related Sterility
Research done on the sterility of items has proven that items remain sterile for long periods of time if the wrapping or container remains intact and is not exposed to events that could render it open to contamination. This is called event-related sterility.
This system defines the sterility of a pack as being dependent on events that may occur during the handling, transportation, and storage of items and sets. A surgical set that has remained intact, after being stored and handled correctly should remain sterile indefinitely.
Events that compromise sterility include:
- Tears or holes in wrappers
- Sterile items that are dropped on the floor
- Securing tapes or locking mechanisms that have been tampered with or removed
- Exposure of packages to blood, fluids, excessive moisture, or extreme temperatures
- Placement of elastic bands to bundle packages together
- Rough handling of packages
- Over-stocking of storage bins that damages packages
Perioperative nurses are responsible for adhering to processes that allow for minimal contact and manipulation of packaged, and sterilized items, as well as the inspection of the integrity of the package prior to opening for use on the sterile field.
Sterilization Indicators – external
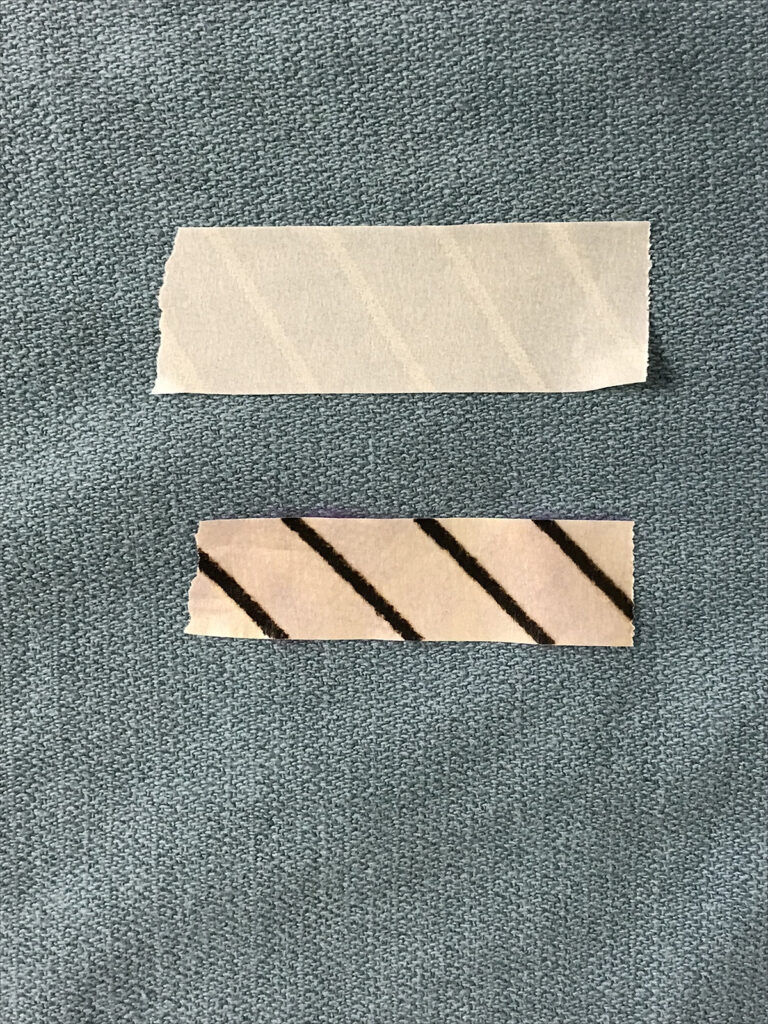
Sterilization indicator tape is used to seal packages during packaging and provides a visual monitor of the sterilization processes externally. These colours and indicator bars may differ according to the manufacturer so perioperative nurses must be familiar with those used in their facility.

Sterilization Indicators – Internal
Internal Chemical indicators are devices placed inside the packaging for sterilization as a second and more thorough indication of sterility. When the perioperative nurse opens the sterile package, the indicator must be verified first before the instrument(s) can be used. The example on the left shows the status of the sterilization process. These indicators vary according to the manufacturer so perioperative nurses must be familiar with those used in their facility.

Effective Yet Environmentally Sustainable Sterilization Processes
Reducing the Environmental Impact of Sterilization Packaging
A Comparative Life Cycle Assessment of Disposable versus Reusable Systems
Read Article
For Surgical Instruments in the Operating Room: A Comparative Life Cycle Assessment of Disposable versus Reusable Systems; watching the videos and reviewing your text, it is hoped that you can recognize that packaging and monitoring of the sterilization process for operating room equipment and instruments are a lot more complex than it initially appeared. Moving forward, perioperative nurses must remain aware of, and advocate for, sterilization processes that are streamlined, effective, and environmentally sustainable.
🧩 Practice Activity
Use the previous and next buttons to navigate through questions. Select “Check” when you feel confident in your answer. When you have completed all the questions, select the “Finish” button.
Tears in Sterilization Wrappers
All surgical supplies must have their sterilization wrappers checked for tears before opening them onto the sterile field. What happens when that step is overlooked?
Case Examples: