Decontamination
All supplies, equipment, and instruments needed for a case are opened in the operating room to be used with a specific patient. Once the patient enters the operating room or the sterile surgical field, the opened items must be used and then discarded or reprocessed as indicated. If the patient enters the room and the case is cancelled without using any of the supplies, the space must still be completely turned over.
The reprocessing of medical devices is one of the most complex yet essential processes in infection prevention for the surgical patient. Items that come into patient contact are categorized, then appropriate processes are followed to ensure that these items are decontaminated (cleaned and disinfected), and when indicated, packaged and sterilized. This module reviews the steps in this process, where they take place, and employee precautions when performing these functions.
Source: Infection prevention orientation manual
Surgical supplies, equipment, and instruments can be either single-use or reusable. Single-use items must be discarded at the end of the surgical procedure.
Reusable items must go through a rigorous process of cleaning & high-level disinfection (decontamination), inspection, packaging, sterilization, and finally correct storage.
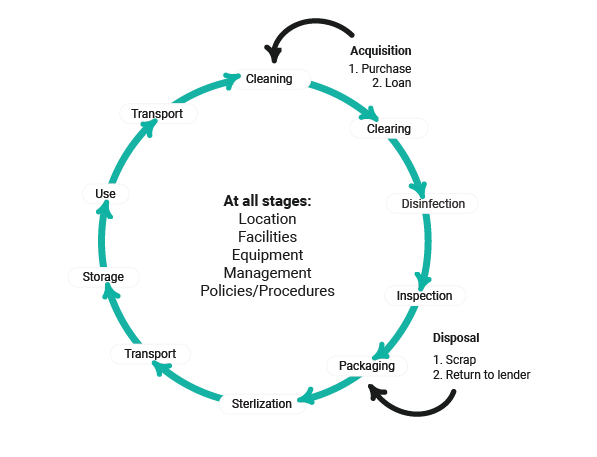
The Decontamination Process
In the surgical setting, decontamination is the combined process of cleaning and disinfecting re-useable medical devices, particularly for perioperative nurses, reusable surgical instruments.
The Decontamination Process: Begins with the Perioperative Nurse
This high-level decontamination renders instruments safe to handle and prepare for sterilization. The physical act of cleaning starts in the operating room where a scrub nurse must keep instruments and equipment free of debris and blood using a cloth and sterile water. This process maintains effective instrument use during surgery.
The Decontamination Process: In Medical Device Reprocessing Department (MDRD)
Postoperative cleaning is performed in the Medical Device Reprocessing Department (MDRD) by qualified technicians. Instruments may go through a mechanical cycle of chemical washing and high temperatures that removes and inactivates, and depending on the microorganism, can destroy pathogens so instruments and other equipment, like stainless steel basin sets, are safe for handling by personnel and are no longer capable of transmitting infectious particles. Specific types of instruments, such as those with complex mechanisms or lumens, go through a dual process of manual cleaning plus a mechanical cycle.
Once the cleaning and disinfection of instruments are completed, they pass through the “contaminated” side of the automated washer-decontaminating unit of the Medical Device Reprocessing Department to the “clean” inspection and packaging area.
(PIDAC, 2013)
📽️ AORN CINE-MED VIDEO
Navigate to the AORN Cine-Med website and make sure that you are logged in before clicking on the link below.
- Sterilization of Reusable Medical Devices in the Health Care Setting (0:49)
- Care and Cleaning of Surgical Instruments
🧩 Practice Activity
Use the previous and next buttons to navigate through questions. Select “Check” when you feel confident in your answer. When you have completed all the questions, select the “Finish” button.

